In this article you will learn ways to get the maximum lifetime of an oxygen sensor in 5 very easy steps.
- Step 1: Ensure Sensor and Interface Electronics Are Set Up Correctly
- Step 2: Assess Environment the Sensor Will Be Used In
- Step 3: Avoid Using the Sensor With Silicones
- Step 4: Protect from Gases and Chemicals That Could Harm the Sensor
- Step 5: Avoid Reducing Atmospheres, Fine Dust and Vibrations
Step 1: Ensure Sensor and Interface Electronics are Set Up Correctly
Commissioning Checks
- Verify the oxygen sensor unit is mounted securely and sealed correctly if appropriate
- If fitted, ensure any baffles are installed in the correct position
- Verify the oxygen sensor and wiring are all undamaged
- Ensure the cables are strain-free and not twisted
- Ensure the oxygen sensor is connected properly, with all its inputs and outputs complete. If appropriate, all screw terminals are properly tightened.
- Test the power supply to ensure it is delivering the correct voltage before wiring to the device.
- Failure to test the suitability of the power supply BEFORE first power on could result in irreversible product damage.
For more information on setting up the sensor, please refer to the Zirconia Operating Principle and Construction Guide sensors.
Step 2: Assess Environment the Sensor Will Be Used In
The application in which the zirconium dioxide oxygen sensor is operating influences the oxygen sensor lifespan.Fail Safe Operation and Sensor Asymmetry
One of the main benefits of the dynamic and active cell employed within the oxygen sensor is that it is inherently fail safe. The continual cycling and measurement of the generated Nernst voltage is effectively the heartbeat of the sensor, if this stops something fatal has occurred within the cell. This can be very quickly detected by the interface electronics.Operating in Aggressive Humid Environments – What Causes An Oxygen Sensor to Fail?
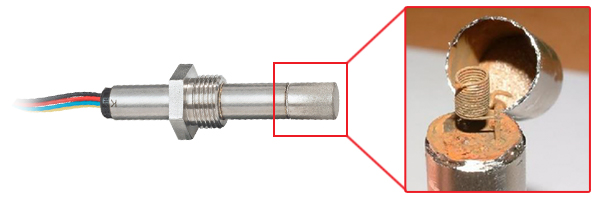
Failure to adhere to these rules will seriously effect the lifetime of an oxygen sensor and result in condensation forming on the heater and sensing element. When the sensor is re-powered the condensation will evaporate, leaving behind corrosive salts which very quickly destroy the heater and sensing element as illustrated. Note how the sensor’s external metalwork looks completely normal.
Protecting from Excessive Moisture
In environments where excessive moisture or falling water droplets are likely, the sensor should be protected from water reaching or falling directly onto the very hot sensor cap as this can cause massive temperature shocks to the cell and heater. Popular methods include a hood over the sensor cap or for the sensor to be mounted in a larger diameter cylinder.At a very minimum the sensor cap should be angled downwards in the application as this will deflect any falling moisture and prevent the sensor cap from filling with water.
Step 3: Avoid Using the Sensor With Silicones
Zirconium dioxide oxygen sensors are damaged by the presence of silicone in the measurement gas. Vapours (organic silicone compounds) of RTV rubbers and sealants are the main culprits and are widely used in many applications. These materials are often made of cheaper silicones, that when heated still outgas silicone vapours into the surrounding atmosphere. When these vapours reach the sensor, the organic part of the compound will be burned at hot sensor parts, leaving behind a very fine divided Silicon Dioxide (SiO2). This SiO2 completely blocks the pores and active parts of the electrodes. If RTV rubbers are used we advise using high quality, well cured materials. Guidance can be provided on request.
Step 4: Protect from Gases and Chemicals Could Harm the Sensor
Combustible Gases
Small amounts of combustible gases will be burned at the hot Pt-electrode surfaces or AI2O3 filters of the sensor. In general, combustion will be stoichiometric as long as enough oxygen is available, the sensor will measure the residual oxygen pressure which leads to a measurement error. The sensor is not recommended for use in applications where there are large amounts of combustible gases present and an accurate O2 measurement is required as these gases will dramatically affect the lifetime on an oxygen sensor. Gases investigated:- H2 (Hydrogen) up to 2%; stoichiometric combustion
- CO (Carbon Monoxide) up to 2%; stoichiometric combustion
- CH4 (Methane) up to 2.5%; stoichiometric combustion
- NH3 (Ammonia) up to 1500 ppm; stoichiometric combustion
| Hydrogen | up to 2% | stoichiometric combustion |
| Carbon Monoxide | up to 2% | stoichiometric combustion |
| Methane | up to 2.5% | stoichiometric combustion |
| Ammonia | up to 1500 ppm | stoichiometric combustion |
Heavy Metals
Vapours from metals like:- Zn (Zinc)
- Cd (Cadmium)
- Pb (Lead)
- Bi (Bismuth)
Halogen and Sulphur Compounds
Small amounts (< 100ppm) of Halogens and/or Sulphur compounds have no effect on the performance of the oxygen sensor. Higher amounts of these gases will, in time, cause readout problems or, especially in condensing environments, corrosion of sensor parts and affect the lifetime of an oxygen sensor. Gases investigated:- Halogens, F2 (Fluorine), Cl2 (Chlorine)
- HCL (Hydrogen Chloride), HF (Hydrogen Fluoride)
- SO2 (Sulphur Dioxide)
- H2S (Hydrogen Sulphide)
- Freon gases
- CS2 (Carbon Disulfide)
Step 5: Avoid Reducing Atmospheres, Fine Dust and Vibrations
Reducing Atmospheres
Long time exposure to reducing atmospheres may in time impair the catalytic effect of the Pt-electrodes and must be avoided. Reducing atmospheres are defined as an atmosphere with very little free oxygen and where combustible gases are present. In this type of atmosphere oxygen is consumed as the combustible gases are burned.Fine Dust/Heavy Shock or Vibrations
Fine dust (carbon parts/soot) may cause clogging of the porous stainless steel filter and could have an effect on the response speed of the sensor. Heavy shocks or vibrations may alter sensor properties resulting in the need for recalibration.If you would like any information about our Zirconia Oxygen Sensors sensors, please do not hesitate to contact us directly.
Related Products
Screwfit Zirconia Oxygen Sensor
Probe Zirconia Oxygen Sensor
Miniature Zirconia Oxygen Sensor
Cost-Effective Oxygen Transmitter - OXY-FLEX Series
Zirconia Oxygen Sensor System - Combustion
Screened Cable Oxygen Sensor
Zirconia Oxygen Sensor System
Zirconia Oxygen Sensor System - Combustion
PCB Mounted Zirconium Dioxide Oxygen Sensor
Probe Zirconia Oxygen Sensor
Screwfit Zirconia Oxygen Sensor
Flange Pinned Zirconia Oxygen Sensor
PCB Mounted Zirconium Dioxide Oxygen Sensor
Flange Wired Zirconia Oxygen Sensor
Miniature Zirconia Oxygen Sensor
Screened Cable Oxygen Sensor
Zirconia Oxygen Sensor System
Want to see more information like this?
Sign up to one of our Industry newsletters and you’ll receive our most-recent related news and insights all directly to your inbox!
Sign Up
