What are the primary emissions from industrial boilers and incinerators that need control?
In layman’s terms, ‘combustion’ involves burning fuel to generate heat. In chemical terms, combustion is an exothermic reaction of the oxidation of hydrocarbons. During the process, oxygen from the air reacts with the hydrogen in the fuel. As the diagram below shows, perfect combustion would produce heat energy, CO2, and H2O as by-products.
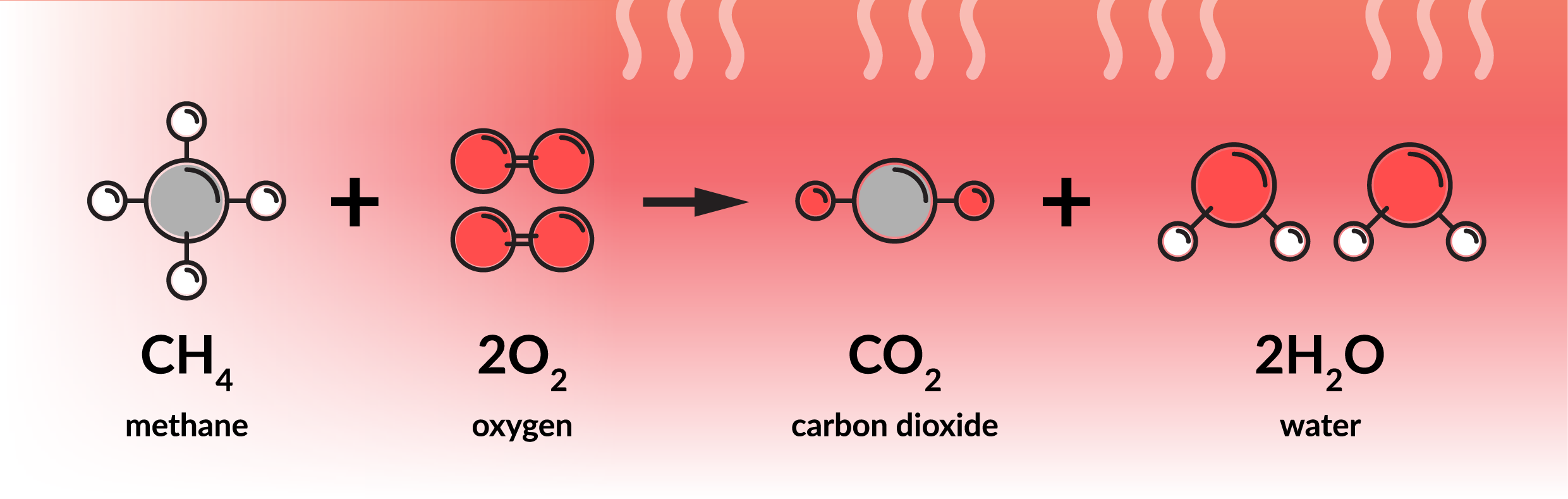
However, controlling the proportions of oxygen and fuel in industrial conditions is complex and inefficient combustion produces some very undesirable pollutants:
NOx – Nitrogen Oxides
Nitric Oxide (NO) and Nitrogen Dioxide (NO2) are two of the most common pollutants in air. They are of concern because they help cause smog and contribute to acid rain.
They are generated in the combustion process as the high temperatures involved cause N2 and O2 molecules to combine. Correctly controlling levels of oxygen in the air feed to the boiler or incinerator helps minimize these compounds.
CO – Carbon Monoxide
Carbon Monoxide is the result of incomplete combustion – this is where not all the energy is released from the fuel because of lack of oxygen. Rather than the carbon atoms joining with two oxygen atoms to form CO2, CO is created instead.
CO is a highly poisonous gas which also contributes to climate change.
Particulates (aka soot)
Soot is simply microscopic solid particles that are generated as part of incomplete combustion in combination with contamination from dust or other elements in the process.
As well as collecting as a black coating on the sides of buildings, these particulates contribute to respiratory diseases.
Note: Since producing CO2 is the aim of the perfect combustion process, we’ve not listed it here as a pollutant. Controlling levels of CO2 emissions is very important, but not in the scope of this post.
Which global regulations control combustion?
Most regions have their own regulations for emissions. The details vary according to the types of industries that are prominent in the country, how developed the nation is, and whether they are part of a wider economic group that enforces general regulations.
Some examples are:
- European Environment Agency Air Quality Standards
- The United States Environmental Protection Agency Air Quality Planning and Standards
- Canada’s Overview of the Multi-Sector Air Pollutants Regulations
- The World Health Organisation issued Air Quality Guidelines in 2005.
A common theme in all these regulations is reducing levels of NOx, CO, and particulate pollutants produced through combustion in incinerators and boilers.
How does monitoring oxygen levels during combustion help?
There are lots of possible reasons for imperfect combustion:
- Wrong air to fuel ratio
- Poor burner performance
- Varying operating conditions
- Varying ambient conditions
- The wear and tear on the burner
The best way to ensure combustion efficiency while decreasing stack heat losses is to keep excess air to a minimum. Monitoring oxygen in the exhaust gas is the most efficient way to determine excess air levels.
Examples of excess air levels
Depending on the fuel, the following levels of excess air are required:
- Natural Gas: minimum 10% of Excess air
- No. 2 Oil: minimum 12% of Excess air
- No. 6 Oil: minimum 15% of Excess air
The amount of excess air depends on the fuel used and often the condition and age of the burner.
Installing a dedicated combustion control analyzer enables users to monitor and fine-tune the excess air levels throughout the combustion process.
Which applications benefit from combustion control analyzers?
Some typical applications include, but are not limited to:
- Power generation using steam boilers
- Thermal crackers in petrochemical
- Glass and steel manufacture
- Incinerators for medical, chemical, and clinical waste
- Crematoria
What to look out for in a combustion analyzer?
The combustion process is highly aggressive, and any analyzer needs to withstand high temperatures and corrosive atmospheres.
Ideally, combustion control analyzers are installed in-situ – with the sensing element placed directly in the flow of emissions in the stack. Although extractive sampling is possible in the application, the results are slower as the sample gas is cooled and filtered before reaching the analyzer.
Zirconium oxide oxygen sensors are ideal for monitoring the exhaust gases for excess air. They can cope with the high temperatures in the application, are resistant to contamination, and have a long sensor life.
What does PST offer for combustion control analysis?
Advanced gas sensors like the OXY-FLEX Oxygen Transmitter from PST, can eliminate elements of inefficiency from your boiler system by measuring the level of oxygen in flue gases and feeding data back to the boiler controller. This enables in situ monitoring of combustion boiler efficiency and regulation of the fuel and air flow input ratios to optimize combustion in real-time.
The gas sensor uses a zirconium dioxide sensing element that can readily withstand the extreme temperatures of boiler flues up to a maximum operating temperature of 400°C (752°F). It can be configured to three distinct output measuring ranges which can be communicated via 4-20mA,0-10Vdc and RS232 outputs through a closed-loop system. This non-depleting oxygen gas sensor provides peace of mind for boiler operators with a service life of up to 10 years.
Have a question about oxygen levels in your combustion process? Contact our support team for assistance.
Want to see more information like this?
Sign up to one of our Industry newsletters and you’ll receive our most-recent related news and insights all directly to your inbox!
Sign Up