
Humidity and temperature control within “hydrogen peroxide (H2O2) industrial processes“
Hydrogen peroxide (H2O2) is a versatile chemical widely used in various industries due to its strong oxidation and disinfectant properties. The main applications include bleaching, water and wastewater treatment, pulp and paper production, chemical synthesis, and decontamination of biohazardous materials. H2O2 has proven to be an effective and safe alternative to commonly used chemicals such as chlorine-based bleaches, with the benefit of less environmental and personal risks from harmful by-products when decomposed.
Decontamination & sterilization processes of biohazardous material
In this blog, we will focus on humidity and temperature control within bio-decontamination and sterilization processes, which is the process of reducing or eliminating the presence of microorganisms in a particular environment or surface, using hydrogen peroxide. This process is typically undertaken in incubators, isolators, laboratories and medical diagnostic devices. We will outline the process and the different phases involved in decontamination and how we can provide a stable solution to measure the effectiveness of the decontamination process!
In bio-decontamination, hydrogen peroxide can be used in various forms, such as vapor, liquid or gas, depending on the type of microorganisms being targeted. One of the advantages is its ability to kill a wide range of microorganisms quickly and effectively, including bacteria, viruses, spores, fungi and biohazardous materials, such as blood, bodily fluids and other organic matter that may be contaminated with pathogens. It works by breaking down into water and oxygen, producing free radicals that fatally damage the cell membranes, DNA and other cell components of all microorganisms.
Vaporized hydrogen peroxide (VHP)
One of the most popular methods of using hydrogen peroxide for bio-decontamination is through a process called vaporized hydrogen peroxide (VHP) sterilization. This process involves generating a fine mist of hydrogen peroxide vapor, which is then circulated throughout the area to be decontaminated. The hydrogen peroxide vapor penetrates all surfaces and kills microorganisms present.
When H2O2 is used as a vaporized decontaminant, the main by-products that can be produced include water (H2O) and oxygen (O2). In theory- these by-products are non-toxic and do not pose a significant risk to human health or the environment:

However, in some cases, other minor by-products, such as hydroxyl radicals (OH) or peracetic acid (PAA) may be formed, depending on the concentration of H2O2 used and the environmental conditions. These by-products are also relatively harmless at the low concentrations typically used for decontamination. Nevertheless, it is essential to properly ventilate and monitor the decontamination process to ensure that any by-product is kept at safe levels.
Decomposition of VHP
Hydrogen peroxide can decompose or disintegrate at different temperatures, depending on various factors such as the concentration of the solution, the presence of catalysts or stabilizers and the duration of exposure to heat. In general, the rate of H2O2 decomposition increases with rising temperatures.
At room temperature, pure hydrogen peroxide may decompose very slowly over time, with a half-life of about one year at 20 °C. However, at higher temperatures, the rate of decomposition increases significantly. For example, at 50 °C, the half-life of 30% hydrogen peroxide solution is about 60 hours!
If hydrogen peroxide is heated rapidly to a high temperature, it can decompose explosively. For example, if heated to its boiling point (150.2 °C) in a sealed container it can rapidly build up pressure and explode.
Therefore, it is important to handle hydrogen peroxide with care and to follow appropriate safety procedures when heating or storing it and to control the humidity and temperature levels.
The 4 phases of a VHP decontamination cycle
The decontamination process can be divided into three phases, preparation phase, decontamination phase and verification phase:
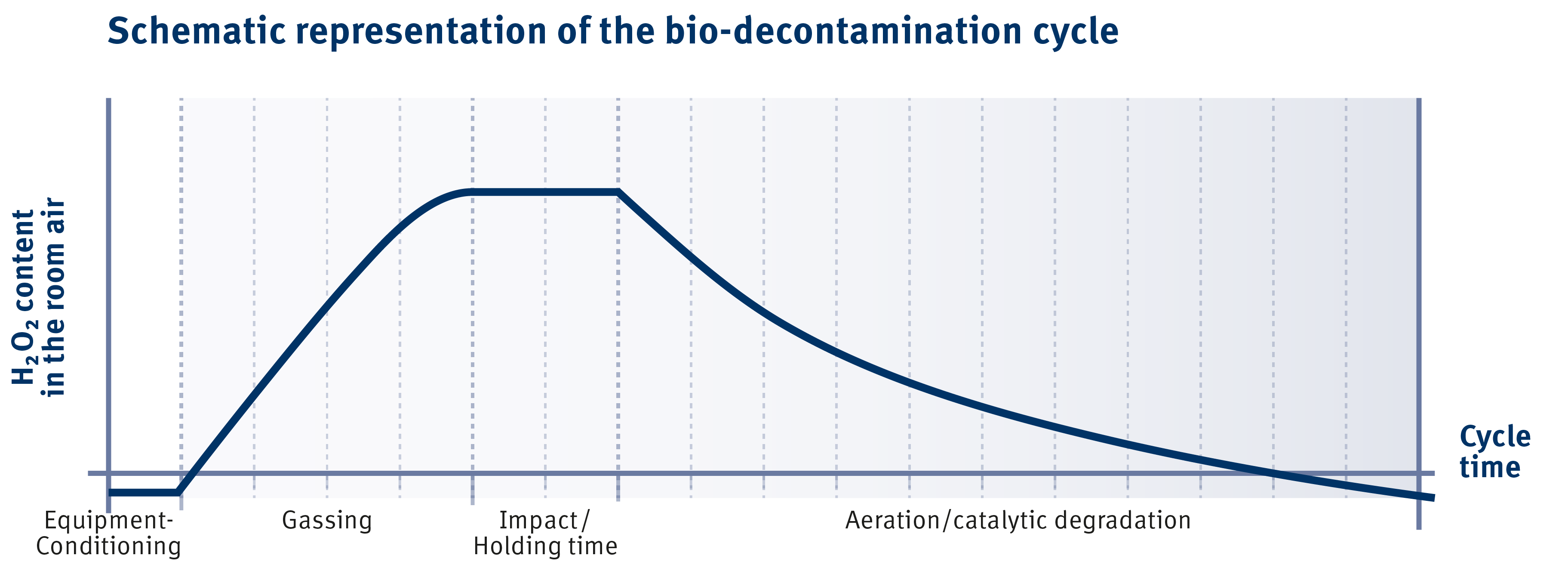
1. Pre-Condition
The area or material to be decontaminated is prepared by controlling the temperature, relative humidity and air quality. This phase ensures that the optimal concentration of hydrogen peroxide is reached for effective decontamination.
2. Dehumidification
Diffusion Phase - H2O2 is introduced into the area or onto the material to be contaminated. This can be done using vapor or aerosolized H2O2. The respective concentration and duration of the decontamination phase depends on the type and amount of contaminants present and the size and complexity of the area or material being decontaminated.
3. Injection: Decontamination
Diffusion Phase - H2O2 is introduced into the area or onto the material to be contaminated. This can be done using vapor or aerosolized H2O2. The respective concentration and duration of the decontamination phase depends on the type and amount of contaminants present and the size and complexity of the area or material being decontaminated.
4. Post-Conditioning
The area or material is now ventilated or the H2O2 catalyzed to reduce the concentration of hydrogen peroxide to safe levels. This is achieved mainly by introducing fresh air, using air filters or catalyzing the H2O2. The duration of this phase depends on the concentration of hydrogen peroxide introduced and the process to condition the air back to safe levels.
Validation of the effectiveness of the decontamination process
If a process is highly repeatable and closely controlled, you can assess the success of an H2O2 decontamination cycle using specific measurements of the humidity and temperature. This is because H2O2 injection with vaporized H2O2 affects the humidity and temperature in a room. An accurate measurement of these parameters before and after the decontamination cycle can help determine whether the desired level has been achieved.
Often processes will also directly measure the H2O2 concentration in the air, using a suitable H2O2 measuring device. However, it is essential to note that measuring the H2O2 concentration in the air may not be sufficient to assess the success of an H2O2 process, as there may be other factors that influence the success, such as the room size, the duration of the sterilization and the concentration of H2O2 used.
Monitoring humidity & temperature
It is important to measure humidity and temperature because hydrogen peroxide is a reactive chemical that can decompose or degrade under certain conditions. High temperatures and humidity can accelerate the decomposition of hydrogen peroxide, leading to a loss of potency and effectiveness. In addition, hydrogen peroxide can react with other substances, such as metals. In the presence of moisture leading to the formation of by-products that can be harmful.
Therefore, it is crucial to control the temperature and humidity in the storage and handling of hydrogen peroxide to ensure its stability and effectiveness. Dehumidifiers or air conditioners can control humidity, and temperature can be controlled by refrigeration or heating systems. Regular monitoring of temperature and humidity levels can help prevent the degeneration of hydrogen peroxide and ensure the quality and safety of the decontamination level.
Rotronic humidity sensor in H2O2 environment
Hydrogen peroxide can potentially degrade or break sensitive materials, such as the polymer used in the humidity sensor and can affect the accuracy and lifespan of the sensor and electronics.
PST offers a special sensor more resistant to H2O2, due to its additional protection cage: the Rotronic HYGROMER® HH-1-SK. This sensor has been proven over 10 years working in conditions exactly as described above. Some of the largest suppliers of H2O2 decontamination equipment have partnered with PST and rely on our sensor technology to ensure their solutions work effectively without fail.
As a final note it is important to remember that careful selection of humidity sensors must be made for the laboratories, isolation rooms and gloveboxes which are routinely sterilized using H2O2. Whilst perhaps not involved in the sterilization process, these control or monitoring sensors will continue to be used. If your sensors are not designed specifically to tolerate repeated exposure to H2O2 they can fail rapidly or provide inaccurate measurements
Contact us to discuss your application and we will be happy to advise suitable products.
Learn more in our statement on how the Rotronic hydrogen peroxide probes work, as well as in the description of a sterilization process:
The Rotronic HYGROMER® HH-1-SK capacitive humidity sensor cannot provide exact water humidity-relevant measurement results in environments containing H2O2 and condensation. In this case, the measurement signals present with condensation are secondarily effected by the H2O2 concentration secondarily.
The humidity sensor itself is H2O2-resistant, meaning the sensor delivers accurate measurement results before and after the H2O2 condensation phase). Correct measured values are shown again after the end of exposure to H2O2.
Despite the Rotronic HYGROMER® HH-1-SK capacitive humidity sensor being tolerant to H2O2 it is still recommended to keep these condensation phases on the sensor element as short as possible. Depending on the application it can be beneficial to permanently remove the protective filter on the probe head and place the probe in a position improved air flow.
Situation description: Sterilization process with H2O2 and measurement of relative humidity
The evaluation of the humidity probes and test results shows that during the injection phase in some process sequences in the sterilization chambers, the rapid introduction of the generated hydrogen peroxide gas-water vapor mixture leads to condensation on the humidity probes.
This is caused by the fact that the probes are exposed to the normal ambient temperature of approx. 22 °C before evaporation of the H2O2. The injection phase is often very fast and ranges from approx. 28 - 30 °C. Due to the injection of H2O2, the relative humidity rises very quickly to values greater than 90 %RH. The thermal mass of the probes causes a delayed increase in the temperature of the probe head. At very high relative humidity, this means a very small difference between the dew point and the probe head temperature. If the dew point temperature reaches the probe head temperature, condensation occurs on all surfaces colder than the dew point temperature.
This means a coating of microdroplets (water with H2O2) forms on the affected areas. The humidity sensor and its electrical connections are also affected by this. The probe then displays a measurement signal that is composed of the actual humidity signal and the additional influences (such as creeping currents in the case of a condensation film (depending on the coat density and specific conductivity), individual drops on the sensor surface, cross-influences due to the formation of galvanic cells in the case of condensation (electrochemical reaction), previous contamination (evaporation residues from previous charging cycles).
Regarding the measurement signal during the injection and exposure phase, it should also be noted that H2O2 decomposes quickly at higher temperatures and in the case of condensation. This decomposition reaction produces additional water which, when evaporating or generated directly in the gaseous phase, causes an additional moisture content. This then leads to higher relative humidity values than would be expected. This condensation phase is maintained until it is physically possible for the water film to dry again. In dry state and during the ventilation phase, the humidity sensor then detects the amount of water vapor in the gaseous phase (standardized to relative humidity according to WMO) correctly again.
Concentrations and pollutants

Related Blogs
Importance of Humidity Measurement in Environmental Chambers
Optimizing Chamber Humidity Control
Measurement Solutions for Environmental Test Chambers
Related Categories
Our Humidity Measurement Products and the Current Catalogue
Overview of Products and Sensors to Measure Relative Humidity
Life Sciences & Pharmaceuticals
Related Products
Probe for Hydrogen Peroxide Environments H2O2 – Rotronic HC2A-S(M)-HH
Ambient Oxygen Analyzer - Ntron Gasenz
Want to see more information like this?
Sign up to one of our Industry newsletters and you’ll receive our most-recent related news and insights all directly to your inbox!
Sign Up
