What are the main sources of hydrogen for industry?
Much of the hydrogen (H2) produced for industry comes from fossil fuels. Steam reforming is a thermochemical process whereby a fossil fuel is heated with water to produce hydrogen and carbon dioxide. ‘Blue’ Hydrogen is produced from natural gas reforming, and ‘brown’ hydrogen from gasified coal.
Both these methods produce carbon dioxide (CO2) as a by-product, which is either released to the atmosphere or collected for use in another process (such as food and drink processing).
There is now a marked trend to move away from the production of hydrogen (H2) via the reduction of natural gas, towards electrolysis of water. In many countries, the power used to operate electrolyzers comes from solar, wind and hydro, as well as from bio-methane fuelled power generation.
Hydrogen produced using renewable energy is termed ‘green’. Although electrolysis requires a lot of energy, renewables like wind and solar don’t emit CO2 like the blue- and grey-produced hydrogen.
Hydrogen can also be produced via biomass gasification. This involves high temperatures (700 °C), but without combustion. The quantities of O2 and steam in the process are controlled to produce CO & H2. This gas, syngas, is then used to power turbines via combustion.
Though growing biomass removes CO2 from the atmosphere, this process needs to be combined with carbon capture to keep the net emissions low.
The electricity required for electrolysis can be up to 75 % of the cost of production of hydrogen, hence the trend towards renewable sources.
How are developments in electrolyzer driving production of green hydrogen?
Electrolyser technology is rapidly advancing and replacing the production of hydrogen using fossil fuels, which is reducing the amount of CO2 produced as a by-product. The growth in this industry is driven by the requirements of various governments to reduce the use of fossil fuels for power generation and transportation, replacing them with fuel cell technology. Fuel cells convert hydrogen and oxygen into electricity and the by-product water. Heat is also generated in this process. That heat in larger facilities can be used to drive steam turbines for power generation – known as ‘co-generation’.
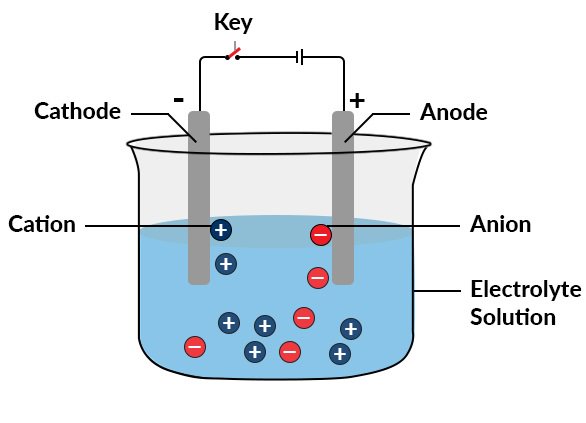
Put simply, electrolysis is the passing of DC current through an electrolyte, resulting in a chemical reaction at the anode and cathode. O2 is produced by oxidation at the anode and H2 by reduction at the cathode.
Electrolysis has been with us since 1800, when Alessandro Volta developed the early electrical pile using acid as a medium, and it was noticed that, when current flowed, oxygen and hydrogen appeared at the poles of the pile. Further research was carried out by Sir Humphry Davy (of the Davy safety lamp fame) and his then assistant Michael Faraday (who formulated two laws of electrolysis)
Alkaline electrolyzers (AEL)
It was found that pure water is not always a good medium for electrolysis. For that reason, modern electrolyzers will use potassium and sodium hydroxide, which offer better reactions. See Electrolysis of water - Wikipedia
The hydroxide ions travel from the cathode to the anode through the electrolyte. Hydrogen is generated on the cathode and oxygen at the anode. This method of production is termed ‘alkaline electrolysis’ and works at a temperature range of 70…90 °C, at pressures of around 30 bar.
PEM electrolyzers
Another type is the polymer electrolyte membrane (PEM) electrolyzer, which uses water to react at the anode to form O2 and positively charged hydrogen ions which move across the ion-conducting membrane to the cathode. The membrane is a special solid polymer material.
These ions then recombine with the external electrons running through the circuit to produce hydrogen gas. Thus, O2 is produced at the anode and H2 at the cathode. This technology produces very pure hydrogen.
Developing other methods of hydrogen production
Photoelectrochemical (PEC) and Photobiological: These processes use light energy to split water into H2 and O2, and are mainly experimental at present.
PEC utilizes panels similar to photovoltaic cells immersed in an electrolyte, with the Sun providing the energy to make the water-electrolyte splitting happen.
For photobiological generation, green microalgae or cyanobacteria use sunlight to split water into hydrogen and oxygen ions.
Process Sensing Technologies’ products for hydrogen and oxygen production
We offer a wide selection of products to ensure both hydrogen and oxygen quality in various stages of the process.
At the outlet of the electrolyzers:
For measuring H2 in O2: The Michell XTC601 This is capable of operating at the production level, which can be a wet process.
For measuring O2 in H2: The Michell XTP601 Both the XTP & XTC can be SIL2-capable.
Where the gas has been dried, the operators want to see low ppm O2 and dry dew points; <10 ppmV and -50°C and below are typical requests.
We recommend:
The Michell Easidew PRO XP Explosion-Proof Dew-Point Transmitter, with a measurement range from -110 up to +20 °C dew point.
The Michell XTP601 Oxygen Analyzer offers a choice of ranges, from 0…0.5 % O2 up to 90…100 % O2.
For high-purity H2, the producers often want to know both how dry the gas is and that the oxygen levels are below certain ppm limits. For this we can offer the Michell Easidew Pro I.S. and the Ntron Minox-i ppm sensor.
For monitoring trace gas in hydrogen, the LDetek HyDetek is a good choice. This instrument is capable of measuring trace impurities in hydrogen down to low parts per trillion, to meet ISO 14687 Part 2 for Hydrogen for use in fuel cells. It can also measure N2 in Hydrogen for leak check and to ensure there is no residual N2 during pipes purging.
Sources:
Hydrogen Production Processes | Department of Energy
Methods of Producing Hydrogen at Scale | Royal Society
Different Types of Electrolyzers - Greendrogen renewable hydrogen
Related Products
Binary Gas Analyzer for Hydrogen Monitoring - Michell XTC601
Oxygen Analyzer - Michell XTP601
Explosion Proof Moisture Transmitter - Easidew PRO XP
Transmitter for Moisture Analysis - Michell Easidew PRO I.S.
Intrinsically Safe Compact Oxygen Transmitter - Minox-i
Turnkey gas system for H2 quality control and monitoring- HyDetek
Quality and Safety Gas Analyzer for Hydrogen Electrolyzer
Want to see more information like this?
Sign up to one of our Industry newsletters and you’ll receive our most-recent related news and insights all directly to your inbox!
Sign Up