INTRODUCTION
This blog describes the physics and concepts behind PST’s range of dynamic and highly accurate oxygen sensors and explains how they can be used to measure humidity.
When reading this blog, keep in mind the following key differentiators between PST’s range of sensors and other zirconium dioxide oxygen sensors:
BACKGROUND PHYSICS
1.1 Partial pressure
1.1.1 Definition
The partial pressure is defined as the pressure of a single gas component in a mixture of gases. It corresponds to the total pressure which the single gas component would exert if it alone occupied the whole volume.
1.1.2 Dalton’s law
The total pressure (Ptotal) of a mixture of ideal gases is equal to the sum of the partial pressures (Pi) of the individual gases in that mixture.
| k |
| i=1 |
From Equation 1 it can be derived that the ratio of the number of particles (ni) of an individual gas component to the total number of particles (ntotal) of the gas mixture equals the ratio of the partial pressure (Pi) of the individual gas component to the total pressure (Ptotal) of the gas mixture.
| ni |
| ntotal |
| Pi |
| Ptotal |
| ni | Number of particles in gas |
| ntotal | Total number of particles |
| pi | Partial pressure of gas i |
| Ptotal | Total pressure |
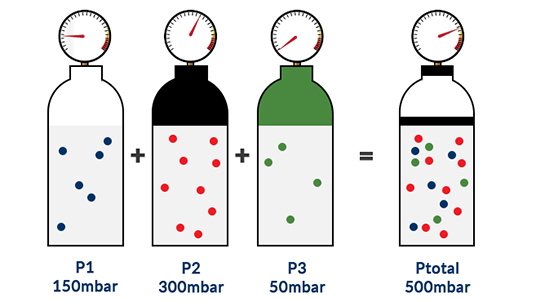
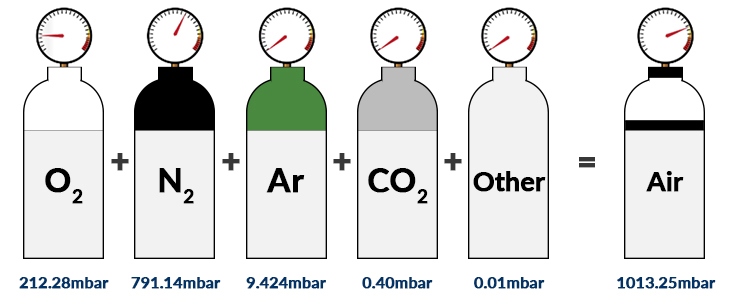
Example 1:
The atmospheric pressure at sea level (under standard atmospheric conditions) is 1013.25mbar. Here, the main components of dry air are nitrogen (78.08% Vol.), oxygen (20.95% Vol.), argon (0.93% Vol.) and carbon dioxide (0.04% Vol.). The volumetric content (%) can be equated to the number of particles (n) since the above gases can be approximated as ideal gases.
Equation 2 can be solved for the partial pressure of an individual gas (i) to get:
| ni |
| ntotal |
The oxygen partial pressure then equates to:
| 20.95% |
| 100% |
Of course, this value is only relevant when the atmosphere is dry (0% humidity). If moisture is present a proportion of the total pressure is taken up by water vapour pressure. Therefore, the partial oxygen pressure (ppO2) can be calculated more accurately when relative humidity and ambient temperature are measured along with the total barometric pressure.
Firstly, water vapour pressure is calculated:
| HRel |
| 100 |
| WVP | Water Vapour Pressure (mbar) |
| HRel | Relative Humidity (%) |
| WVPmax | Maximum Water Vapour Pressure (mbar), which depends on temperature |
For a known gas temperature, the maximum water vapour pressure (WVPmax) can be determined from the lookup table in APPENDIX A. The maximum water vapour pressure is also referred to as the dew point. Warmer air can hold more water vapour and so has a higher (WVPmax) than colder air.
Partial oxygen pressure then equates to:
| 20.95 |
| 100 |
| ppO2 | Partial Pressure O2 (mbar) |
| (Ptotal) | Total Pressure (mbar) which is the same as barometric pressure when we are talking about ambient air. |
| WVP | Water Vapour Pressure (mbar) |
Example 2 below describes the effect of humidity reducing the partial oxygen pressure and therefore the volumetric content of oxygen.
Example 2:
On a typical day, the following information is recorded from a calibrated weather station:
| Temperature | 22°C |
| Humidity | 32% |
| Barometric Pressure Ptotal | 986mbar |
Using the lookup table in APPENDIX A, WVPmax = 26.43mbar.
| 32 |
| 100 |
Partial oxygen pressure then equates to:
| 20.95 |
| 100 |
Because we now know the oxygen partial pressure and the total barometric pressure, we can work out the volumetric content of oxygen.
| 204.8 |
| 986 |
Measuring Humidity in Air
We have seen that the PST oxygen sensors measure one component, ppO2, of a gas mixture. If we also know Ptotal then we can calculate the percentage of oxygen in the mixture. For a known gas mixture, such as air, if we detect a reduction in the oxygen concentration, it can only be because some other gas has been introduced and has diluted the gases which are contained in air.
In this way, the humidity of the gas can be inferred, and we can use the oxygen sensor as a humidity sensor. The benefit of using PST’s oxygen sensors to measure humidity, over other sensors which directly measure humidity is the ability to measure at high temperature. Humidity sensors based on capacitance, for example, are generally limited to operation at low temperatures (<100oC). PST’s oxygen sensors are able to operate in gas temperatures up to 400 oC.
By rearranging the above equations, we can show that:
| 100 |
| WVPmax |
| 100 | |
| 20.95 |
As long as we know that the gas in question is air and that the only thing which has been added is water, then equation 7 is valid. Therefore, this method can be used to measure humidity in applications such as steam cooking, baking and drying.
This method of measuring humidity is simple, because the gas mixture we call “air” is abundant, pervasive and consistent, wherever we are on the planet.
The lookup table in APPENDIX A is limited to temperatures up to 130oC. In order to make calculations above this value, other tables or equations should be used.
Measuring Humidity in Other Gases
When the gas is not air, the calculation of humidity from oxygen becomes more complex. The reduction of oxygen pressure indicates that the gas constituents have changed but we may not know why or how. Take, for example, the process of combustion. Rather that the simple and proportional dilution of air (O2, N2, CO2, Ar etc.) by the introduction of H2O; in a combustion process H2O is produced along with CO2, while O2 and the hydrocarbons (CxHy) which were burned are reduced.
Only with a measurement of the other residual gases, including the O2, can the humidity be calculated.
APPENDIX A – WATER VAPOUR PRESSURE LOOKUP TABLE
Lookup table for maximum water vapour pressure.
| Temperature (°C) | Max water vapour pressure (mbar) | Temperature (°C) | Max water vapour pressure (mbar) |
| 0 | 6.1 | 31 | 44.92 |
| 1 | 6.57 | 32 | 47.54 |
| 2 | 7.06 | 33 | 50.3 |
| 3 | 7.58 | 34 | 53.19 |
| 4 | 8.13 | 35 | 56.23 |
| 5 | 8.72 | 36 | 59.42 |
| 6 | 9.35 | 37 | 62.76 |
| 7 | 10.01 | 38 | 66.27 |
| 8 | 10.72 | 39 | 69.93 |
| 9 | 11.47 | 40 | 73.77 |
| 10 | 12.27 | 42.5 | 84.19 |
| 11 | 13.12 | 45 | 95.85 |
| 12 | 14.02 | 47.5 | 108.86 |
| 13 | 14.97 | 50 | 123.86 |
| 14 | 15.98 | 52.5 | 139.5 |
| 15 | 17.04 | 55 | 457.42 |
| 16 | 18.17 | 57.5 | 177.25 |
| 17 | 19.37 | 60 | 199.17 |
| 18 | 20.63 | 62.5 | 223.36 |
| 19 | 21.96 | 65 | 250.01 |
| 20 | 23.37 | 67.5 | 279.31 |
| 21 | 24.86 | 70 | 311.48 |
| 22 | 26.43 | 75 | 385.21 |
| 23 | 28.11 | 80 | 473.3 |
| 24 | 29.82 | 85 | 577.69 |
| 25 | 31.66 | 90 | 700.73 |
| 26 | 33.6 | 95 | 844.98 |
| 27 | 35.64 | 100 | 1013.17 |
| 28 | 37.78 | 110 | 1433.61 |
| 29 | 40.04 | 120 | 1988.84 |
| 30 | 42.42 | 130 | 2709.58 |
APPENDIX B – SPECIAL NOTES AND APPLICATION HINTS
To ensure the best performance from your equipment it is important that the attached oxygen sensor is installed and maintained correctly.
Document Zirconia Sensor Operation and Compatibility Guide provides some essential sensor operating tips and a complete list of gases and materials that MUST be avoided to ensure a long sensor life or book a call with our technical experts at oxygen@processsensing.com.
Related Products
Screwfit Zirconia Oxygen Sensor
Miniature Zirconia Oxygen Sensor
Probe Zirconia Oxygen Sensor
Want to see more information like this?
Sign up to one of our Industry newsletters and you’ll receive our most-recent related news and insights all directly to your inbox!
Sign Up
